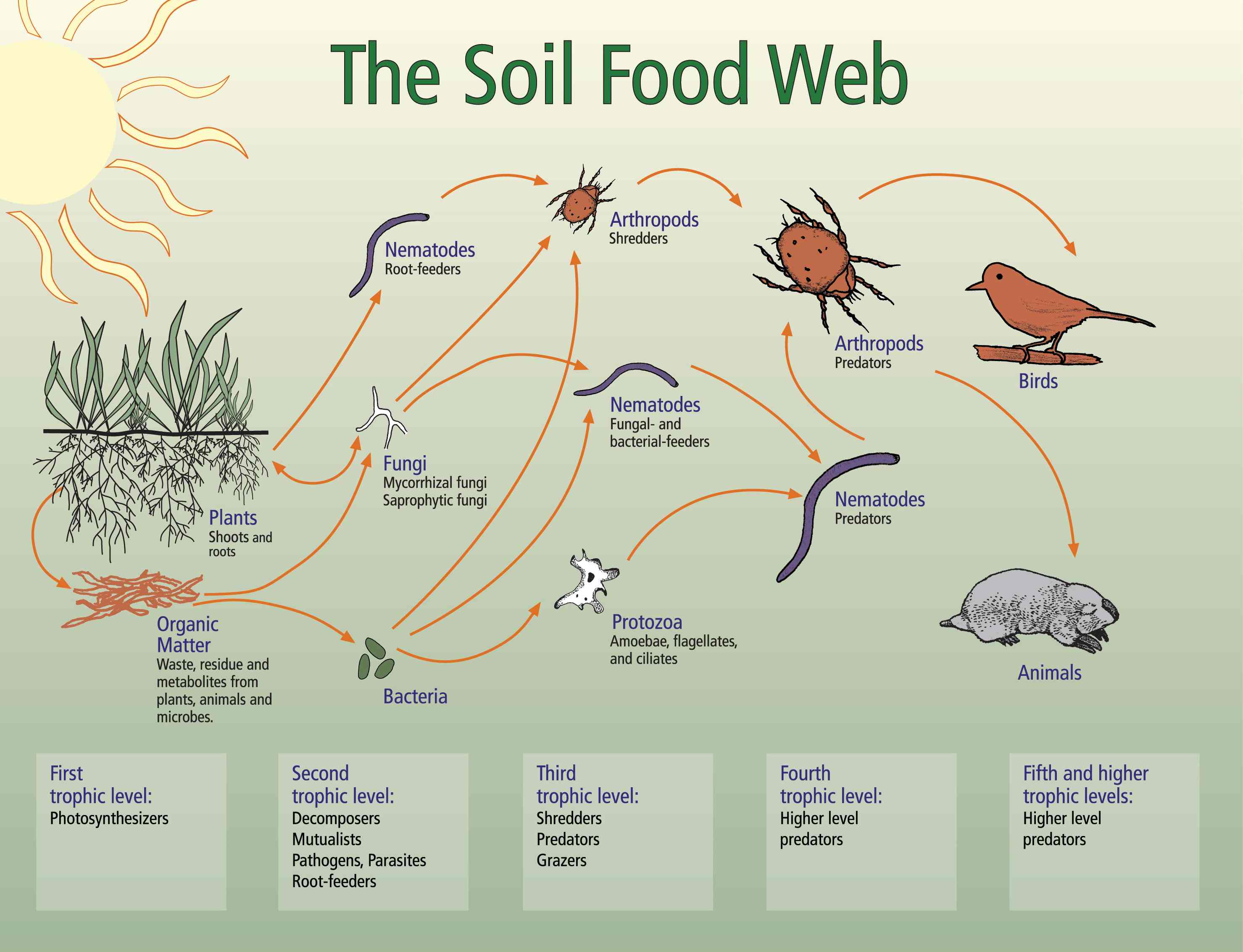
The Soil Foodweb
| The Soil Foodweb and it's Importance in Ecosystem Health |
The structure and function of the soil foodweb has been suggested as a prime indicator of ecosystem health and problems associated with current agricultural and economic development. (Coleman, et al. 1992; Klopatek, et al. 1993).
Measurement of disrupted soil processes, decreased bacterial or fungal activity, decreased fungal or bacterial biomass, changes in the ratio of fungal to bacterial biomass relative to expected ratios for particular ecosystems, decreases in the number or diversity of protozoa, and a change in nematode numbers, nematode community structure or maturity index, can serve to indicate a problem long before the natural vegetation is lost or human health problems occur (Bongers, 1990; Klopatek et al. 1993).
Soil ecology has just begun to identify the importance of understanding soil foodweb structure and how it can control plant vegetation, and how, in turn, plant community structure affects soil organic matter quality, root exudates and therefore, alters soil foodweb structure. Since this field is relatively new, not all the relationships have been explored, nor is the fine-tuning within ecosystems well understood.
Regardless, some relationships between ecosystem productivity, soil organisms, soil foodweb structure and plant community structure and dynamics are known, and can be extremely important determinants of ecosystem processes (Ingham and Thies, 1995). Alteration of the soil foodweb structure can result in sites which cannot be regenerated to conifers, even with 20 years of regeneration efforts (Perry, 1988; Colinas et al, 1993). Work in intensely disturbed forested ecosystems suggests that alteration of soil foodweb structure can alter the direction of succession. By managing foodweb structure appropriately, early stages of succession can be prolonged, or deleted (Allen and Allen, 1993). Initial data indicates that replacement of grassland with forest in normal successional sequences requires alteration of soil foodweb structure from a bacterial-dominated foodweb in grasslands to a fungal-dominated foodweb in forests (Ingham, E. et al, 1986 a, b; 1991; Ingham and Thies, 1995).
In addition to responses to disturbance, it is clear that species diversity, community diversity and foodweb complexity increases with increasing successional stage (Moore et al., 1991; Ingham, E. et al., 1989). Indeed, examination of foodweb interactions and ecosystem diversity, instead of community diversity, may result in new ecosystem measures which reflect this increased community diversity and increased connectivity in later successional stages. The numbers, biomass, activity and community structure of the organisms which comprise the soil foodweb can be used as indicators of ecosystem health because these organisms perform critical processes and functions. Soil decomposers (bacteria, fungi and possibly certain arthropods) are responsible for nutrient retention in soil. If nutrients are not retained within an ecosystem, future productivity of the ecosystem will be reduced as well as cause problems for systems into which those nutrients move, especially aquatic portions of the landscape (Hendrix et al, 1986; Klopatek, et al. 1993).
As ecosystems become more productive, the total amount of nutrients retained within the system increases. As succession occurs, nutrients are increasingly immobilized in forms that are less available for plants and animals, such as phytates, lignins, tannins, humid and fulvic acids (Coleman et al, 1985, 1992). In order for nutrients to become available once again to plants and animals, they must be mineralized by the interaction of decomposers, i.e. bacteria and fungi, and their predators, i.e. protozoa, nematodes, microarthropods, and earthworms (if present). These predator populations and the rates at which they perform mineralization processes are important to ecosystem stability.
The activity of these predator-prey interactions (which determines the rate at which mineralization occurs) are in turn affected, and perhaps controlled by, higher level predators such as millipedes, centipedes, beetles, spiders, and small mammals. It is perhaps something of a conundrum that in healthy ecosystems, while nutrient cycling and productivity increases, nutrient loss is minimized. What makes this possible is the increasing complexity of the soil foodweb. As total ecosystem productivity increases, biodiversity below ground, i.e., the structure and function of the soil foodweb, also increases (Moore et al. 1991). The greater the foodweb complexity, i.e., the interaction of decomposers, their predators, and the predators of those predators responsible for nutrient cycling and the retention of nutrients within the soil (Coleman et al, 1985; 1992), the fewer the losses of nutrients from that system, the more tightly nutrients cycle from retained forms to plants, and back again. Without the soil foodweb, plants would not obtain the nutrients necessary for growth, and the above ground foodweb would not long continue (Nannipieri et al. 1990).
Interactions of decomposers with their predator groups (protozoa, nematodes and microarthropods) maintain normal nutrient cycling processes in all ecosystems (Coleman 1985, 1992). Plant growth is dependent on microbial nutrient immobilization and soil foodweb interactions to mineralize nutrients (Nannipieri et al. 1990). In undisturbed ecosystems, the processes of immobilization and mineralization are tightly coupled to plant growth. Following disturbance, this coupling is lost or reduced (Ingham et al. 1986a, b; Coleman et al., 1992).
By monitoring soil organism dynamics, we can detect detrimental ecosystem changes and possibly prevent further degradation (Lal and Stewart, 1992). The response of each group of soil organisms, i.e., soil saprophytic bacteria, symbiotic bacteria, saprophytic fungi, mycorrhizal fungi, protozoa, and nematodes, with respect to their total biomass and activity, can be used to indicate effects of contaminants on soil health. Instead of relying on an indirect measure of whether total biomass or activity is reduced (e.g., Paul and Clark, 1990; Nannipieri et al. 1991), active and total biomass of each organism group can be directly measured (Ingham et al. 1986).
Lal and Stewart (1992) reviewed the relationship between system health and soil organic matter, and suggested that soil organism losses correlate with detrimental ecosystem changes. Development of the relationship between soil foodweb structure and function and assessment of potential toxic impact could be extremely useful for assessing ecosystem health.
Two measures of ecosystem processes are discussed below: the ratio of fungal to bacterial biomass (Ingham and Horton, 1987) and the Maturity Index for nematodes (Bongers, 1985). Both appear to be useful predictors of ecosystem health, although they must be properly interpreted given the successional stage being examined. For example, recently disturbed systems have nematode community structures skewed towards opportunistic species and genera, while the less opportunistic, more K-selected species of nematodes return as time since-disturbance increases. Thus, healthier soils tend to have more mature nematode community structures. However, as systems mature, nutrients tend to be more sequestered in soil biomass and organic matter, and thus the maturity index reflects an optimal, intermediate disturbance period in which greatest ecosystem productivity is likely to occur.
Ratios of fungal to bacterial biomass also predict this type of response. Highly productive agricultural soils tends to have ratios near one, but asa system undergoes succession into a grassland, this ratio dips downwards, indicating that for a healthy grassland system, the ratio should be less than one. In other words, bacterial-biomass dominates in healthy grassland soils. However, as succession proceeds yet further, fungal biomass begins to dominate and healthy forest systems have fungal to bacterial biomass ratios of greater than one, usually greater than 10.
Piparian or deciduous forests appear to be intermediate within this range of values. Alder forest soils are dominated by bacterial biomass, while popular forest soils are fungal-dominated. Clearly, further investigation is required.
The predators of bacteria and fungi tend to follow the dominance of the decomposer groups. Thus, bacterial-dominated soils have a majority of bacterial-predators (protozoa and bacterial-feeding nematodes), while fungal-dominated soils have a majority of fungal predators (fungal-feeding nematodes and fungal-feeding microarthropods).
Much work is still required at the bacterial and fungal species level.While the species of protozoa and nematodes have been researched in soils of this area of the west, publication of much of this information has yet to occur. Up-dates will be required as this information becomes available.
The Soil Foodweb: Function
Detrital food web in shortgrass prairie
Bacteria and fungi perform one of the major nutrient cycling processes, nutrient retention, in soil (Coleman et al. 1992). The amount of N. P. S and other nutrients immobilized in bacterial and fungal biomass can be considerable, from several micrograms to milligrams of biomass, comprising a significant portion of the stable nutrient pool (Ingham et al, 1986). When the bacterial or fungal component of the soil declines, more nutrients are lost into the ground and surface water (Hendrix et al, 1986; Coleman et al., 1992). A major means of retaining nutrients may also be arthropod fecal material (Rusek, 1983; Pawluk, 1983), depending on the ecosystem.
Soil bacteria are important in maintaining normal nutrient immobilization and decomposition processes in all ecosystems (Coleman et al. 1985; Ingham, et al. 1986a, b). Plants are strongly influenced by the presence of bacteria in the rhizosphere, especially with respect to microbial immobilization of nutrient, and mineralization of nutrients from bacterial biomass by predators. Disturbance of these soil processes may result in the un-coupling of mineralization and plant growth, with the resultant loss of nutrients from the system, causing problems for systems into which nutrients move (Ingham and Coleman, 1984).
As climate changes occur, bacterial populations in the soil could be significantly impacted (Coleman et al, 1992). As temperature increases, bacterial numbers could increase, resulting in greater immobilization of nutrients in their biomass, causing greater nitrogen limitation of plant growth. Alternatively, bacteria could be inhibited by increases in carbon dioxide, resulting in decreased decomposition of soil organic matter and plant litter, which ultimately would change soil structure and nutrient cycling. In addition, current work indicates that alterations in the fungal to bacterial biomass ratio strongly impacts vegetative community structure. If a forest soil, usually strongly dominated by fungi, loses the fungal component, reflected by a decrease in the ratio of fungi to bacteria, conifer species may be at risk of death. If the fungal to bacterial biomass ratio decreases past one, re-establishment of conifer species may be impossible.
Saprophytic fungi and bacteria form the base of the detrital foodweb, and as such are critically important for supporting the nutrient cycling sub-system of any ecosystem, landscape, or biome. Bacterial and fungal pathogens of plants, insects, rodents, and other organisms can control the population density of their hosts.
Mutualist bacteria and fungi can be critically important for plants and animals alike, for example, nitrogen-fixing bacteria on legumes, or rumen bacteria in cows, deer or elk. Without their mutualists, these plants and animals are not capable of competing with other organisms and become locally extinct. While methods are not yet capable of distinguishing between saprophytic and pathogenic species of bacteria and fungi in soil, their total and active biomass, and effects of different disturbances on their distributions, can be estimated. However, work should continue on methods to differentiate bacterial and fungal community composition in soil.
Protozoa, comprised of the three groups; (1) flagellates, (2) amoebae (both naked and testate), and (3) ciliated, are important in maintaining plant-available N and mineralization processes (Coleman, 1985) and, as bacterial-feeders, are important in controlling bacterial numbers and community structure in the soil (Foissner 1986). The presence or absence of certain protozoa species is indicative of the presence of certain hazardous wastes and therefore may be highly useful indicator organisms of certain types of environmental impacts (Foissner 1986).
Nematodes are one of the most ecologically diverse groups of animals on earth, existing in nearly every habitat. Nematodes eat bacteria, fungi, algae, yeasts, diatoms and may be predators of several small invertebrate animals, including other nematodes. In addition, they may be parasites of invertebrates, vertebrates (including man) and all above and below ground portions of plants. Nematodes range in length from 82 um (marine) to 9 m (whale parasite) but most species in soil are between 0.25 and 5.5 mm long.
Nematodes are recognized as a major consumer group in soils, generally grouped into four to five trophic categories based on the nature of their food, the structure of the stoma and esophagus and method of feeding (Yeates, 1971). Plant-feeding nematodes possess stylets with a wide diversity of size and structure and are the most extensively studied group of soil nematodes because of their ability to cause plant disease and reduce crop yield. Fungal-feeding nematodes have slender stylets but are often difficult to categorize and have been included with plant-feeders in many ecological studies. Bacterial feeding nematodes are a diverse group and usually have a simple stoma in the form of a cylindrical or triangular tube, terminating in a teeth (Nicholas, 1975).valve-like apparatus which may bear minute nematodes (marine) to 9 m 0.25 and 5.5 mm
Predatory nematodes are usually large species possessing either a large styles or a wide cup-shaped cuticular-lined stoma armed with powerful teeth (Nicholas, 1975). Omnivores are sometimes considered as a fifth trophic category of soil nematodes. These nematodes may fit into one of the categories above but also ingest other food sources. For example, some bacterial feeders may also eat protozoa and/or algae and some stylet-bearing nematodes may pierce and suck algae as well as fungi and/or higher plants. Stages of animalparasitic nematodes, such as hookworms, may also be found in soils but generally are not common in most soil samples.
Nematodes and protozoa function as regulators of mineralization processes in soil (Coleman, 1985). Bacterial- and fungal-feeding nematodes release a large percent of N when feeding on their prey groups and are thus responsible for much of the plant available N in the majority of soils (Ingham, R. et al. 1985). Nematode-feeding also selects for certain species of bacteria, fungi and nematodes and thereby influences soil structure, carbon utilization rates, and the types of substrates present in soil (Ingham, R. 1992). Root-feeding nematodes are among the greatest pests in agricultural systems and, with the loss of many nematicides, are becoming a greater concern. Without doubt, plant establishment, survival and successional processes are influenced by these soil organisms.
Soil processes are important for maintaining normal nutrient cycling in all ecosystems (Coleman et al., 1985; Dindal 1990; Ingham, E. et al. 1986a, b). Plant growth is dependent on the microbial immobilization and soil foodweb interactions to mineralize nutrients. In undisturbed ecosystems, the processes of immobilization and mineralization are tightly coupled to plant growth but following disturbance, this coupling may be lost or reduced. Nutrients may be no longer retained within the system, causing problems for systems into which nutrients move (Ingham and Coleman, 1984; Hendrix et al. 1986; Nannipieri et al. 1990). Measurement of disrupted processes may allow determination of a problem long before normal cycling processes are altered, before the natural vegetation is lost, or human health problems occur. By monitoring soil organism dynamics, we can perhaps detect detrimental ecosystem changes and possibly prevent further degradation. Immobilization of nutrients in soil, i.e., retention of carbon, nitrogen, phosphorus, and many micronutrients in the horizons of soil from which plants obtain their nutrients, is a process performed by bacteria and fungi. Without these organisms present and functioning, nutrients are not retained by soil, and the ecosystem undergoes degradation. Thus, to assess the ability of an ecosystem to retain nutrients, the decomposed portion of the ecosystem, i.e., active and total fungal biomass, and active bacterial biomass must be assessed.
The Soil Foodweb: Structure
Worksheet for 1 sq. m soil
What is the soil foodweb? Per gram of healthy soil, which is about a teaspoon of soil plus organic matter, the following organisms are found, many of which are mostly unknown to scientists. Bacteria break down easy to-use organic material, and retain the nutrients, like N. P and S. in the soil. About 60% of the carbon in those organic materials are respired as carbon dioxide, but 40% of that carbon is retained as bacterial biomass. The waste products bacteria produce become soil organic matter. This "waste" material is more recalcitrant than the original plant material, but can be used by a large number of other soil organisms, exemplifying the classic statement that "One man's garbage is another's treasure". Productive garden soil should contain more bacteria than any other kind of organism, although care must be taken to make sure beneficial bacteria, instead of disease-causing bacteria, are most prevalent.- S to 60 000 meters of fungal hyphae. Fungi break down the more recalcitrant, or difficult-to-decompose, organic matter, and retain those nutrients in the soil as fungal biomass. Just like bacteria, fungal waste products become soil organic matter, and these waste materials are used by other organisms. Gardens require some fungal biomass for greatest productivity, but in order for best crop growth, there should be an equal biomass of bacteria as compared to fungi. Most grasslands or pastures have less fungi than bacterial, while all conifer forests have much more fungal, as compared to bacterial, biomass. As with bacteria, some fungi cause disease and the soil must be managed to prevent these fungi from being a problem.
-100 to 100,000 protozoa. These organisms are one-celled, highly mobile organisms that feed on bacteria and on each other. Because protozoa require 5 to 10-fold less nitrogen than bacteria, N is released when a protozoan eats a bacterium. That released N is then available for plants to take up. Between 40 and 80% of the N in plants can come from the predator-prey interaction of protozoa with bacteria.
- 5 to 500 beneficial nematodes. Beneficial nematodes eat bacteria, fungi, and other nematodes. Nematodes need even less nitrogen than protozoa, between 10 and 100 times less than a bacterium contains, or between 5 and 50 times less than a fungal hyphae contains. Thus when bacterial- or fungal-feeding nematodes eat bacteria or fungi, nitrogen is released, making that N available for plant growth. However, plant-feeding nematode are pests because they eat plant roots. These "bad" nematodes can be controlled bacteria, fungi, and other nematodes. Nematodes need even less nitrogen than protozoa, between 10 and 100 times less than a bacterium contains, or between 5 and 50 times less than a fungal hyphae contains. Thus when bacterial- or fungal-feeding nematodes eat bacteria or fungi, nitrogen is released, making that N available for plant growth. However, plant-feeding nematode are pests because they eat plant roots. These "bad" nematodes can be controlled biologically, as they are in natural systems, by fungi that trap nematodes, by having fungi that colonize root systems and prevent nematode attack of roots, or by predation of nematodes by arthropods. In cases of extreme outbreaks, however, the only answer may be the use chemicals to control these plant-feeding nematodes. However, once a chemical is used which kills the beneficial nematodes as well as the plant-feeding ones, the beneficial nematodes need to be replaced through inoculation.
- A few to several hundred thousand microarthropods. These organisms have several functions. They chew the plant leaf material, roots, stems and boles of trees into smaller pieces, making it easier for bacteria and fungi to find the food they like on the newly revealed surfaces. The "comminuting" arthropods can increase decomposition rates by 2- to 100- times, although if the bacteria or fungi are lacking, increased decomposition will not occur. In many cases, however, the arthropods carry around an inoculum of bacteria and fungi, making certain the food they want is inoculated onto the newly exposed surfaces! Arthropods then feed on bacteria and fungi, and because the C:N ratio of arthropods is 100 times greater than the bacteria and fungi, they release nitrogen which then is available for plant growth. Some arthropods eat pest insects, while others eat roots. Again, it's important to encourage the beneficial ones and discourage the ones that eat plants!
The Web of Life Can Be Degraded
The interactions between these organisms form a web of life, just like the web that biologists study above ground. What most people don't realize is that the above ground wouldn't exist without the below ground systems in place and functioning. Soil biology is understudied, compared to the above ground, yet it is important for the health of gardens, pastures, lawns, shrub lands, and forests. If garden soil is healthy, there will be high numbers of bacteria and bacterial-feeding organisms. If the soil has received heavy treatments of pesticides, chemical fertilizers, soil fungicides or fumigants that kill these organisms, the tiny critters die, or the balance between the pathogens and beneficial organisms is upset, allowing the opportunist, disease-causing organisms to become problems.
Over-use of chemical fertilizers and pesticides have effects on soil organisms that are similar to over-using antibiotics. When we consider human use of antibiotics, these chemicals seemed a panacea at first, because they could control disease. But with continued use, resistant organisms developed, and other organisms that compete with the disease-causing organisms were lost. We found that antibiotics couldn't be used willy-nilly, that they must be used only when necessary, and that some effort must be made to replace the normal human-digestive system bacteria killed by the antibiotics.
Soils are similar, in that plants grown in soil where competing organisms have been knocked back with chemicals are more susceptible to disease-causing organisms. If the numbers of bacteria, fungi, protozoa, nematodes and arthropods are lower than they should be for a particular soil type, the soil's "digestive system" doesn't work properly. Decomposition will be low, nutrients will not be retained in the soil, and will not be cycled properly. Ultimately, nutrients will be lost through the groundwater or through erosion because organisms aren't present to hold the soil together.
The best way manage for a healthy microbial ecosystem in a home garden is to routinely apply organic material, such as compost. To keep garden soil healthy, the amount of organic matter added must be equal to what the bacteria and fungi use each year.
Indiscriminate use of chemical fertilizers and pesticides should be avoided. If the soil is healthy for the type of vegetation desired, there should be no reason to use pesticides, or fertilizers. If a decision is made to change from grass to garden, or forest to lawn, a massive change in the soil foodweb structure is required and chemical use, along with judicious addition of the right kind of compost with the right kinds of organisms, may be necessary for a few years. But once the correct soil foodweb structure is in place, there should be no reason to apply chemicals.
If both bacteria and fungi are lost, then the soil degrades, than any other organism. If bacteria are killed through pesticide or chemical applications, and especially if certain extremely important bacteria like nitrogen-fixing bacteria or nitrifying bacteria are killed, fungi can take over and crop production can be harmed. For example, current research indicates that the reason moss takes over in lawn ecosystems is because the soil is converted from a bacterial dominated system to one dominated by fungi. nutrients are lost, erosion increases and plant yield is reduced. If inorganic fertilizers are used to replace the lost nitrogen, the immediate effect may be to improve plant growth. However, as time goes on, it is clear that inorganic fertilizers can't replace the other kinds of food that bacteria and fungi need. After awhile, fertilizer additions are a waste of money, because there aren't enough soil organisms to hold on to the nutrients added. Surface and groundwater will become contaminated with the lost nutrients, causing problems.
Maintaining and Enhancing the Soil Foodweb
Bacterial dominance is maintained by mixing plant material into the soil. But the bacteria and fungi eat this material at an amazingly rapid pace and new inputs are required every year; Fungi can be maintained by letting litter accumulate on the soil's surface. Larger soil organisms like millipedes, centipedes, earthworms, and ants mix plant material into soil and open air channels, especially important in wet periods in heavy clay soils. To maintain a one-to-one ratio of bacteria and fungi needed for crop systems, a balance is needed between too much and too little mixing. Plant material needs to be mixed in enough to maintain bacterial dominance, but too much mixing results in soil degradation. Timing of mixing is important as well, but the optimal combination hasn't been determined for soil organisms in different types of soil.
It's important to remember that grassland, garden and forest soils represent a gradient from bacterial to fungal dominance. Gardens require equal amounts of bacteria and fungi, while trees require fungi. There are a number of examples where the fungal component has been lost from forest soils and as a result, tree regeneration is impossible. If the soil foodweb was better understood, there would ways to fix the problem, but that research is yet to be done. In order to determine the organisms in soil, the biomass and activity of bacteria and fungi, the numbers of protozoa and nematodes, the types (beneficial and root-feeding) of nematodes, and VA mycorrhizal colonization of roots need to be assessed. Reference information on the biomass, numbers and types of these organisms is being determined for soils all over the world. The goal is to determine what the healthy soil foodweb structure should be for every soil type, given vegetation and climate characteristics. If the foodweb structure is not at that healthy level, another goal is to determine what it will take to return it to a healthy level. Once a healthy foodweb structure is achieved, the only time testing would be needed is when some problem is detected, suggesting the foodweb has changed in an unproductive direction.
Soil Sampling
Soil sampling should result in three samples from any particular area, such as a meadow, crop field, forest stand or garden. Five samples per area, or more would be preferable, but time and cost of analysis must be a consideration. The idea is to take enough samples that the variability within that area can be assessed. One possible approach is to mentally split the area to be sampled into three equal areas. From each of the three areas, between three and ten small soil cores should be mixed together in a plastic bag. The cores should be taken by pushing aside the litter (loose recognizable plant litter material) on the top of the soil and removing soil (may contain some unrecognizable plant material, but is mostly mineral soil material, or sand, silt and clay fractions) from the 0-5 cm depth. The core should be about 2.5 cm or 1 inch diameter, and all the soil from this small cylinder should be removed and placed in the plastic bag. If mycorrhizal colonization is to be performed, the roots in each core should be removed and placed in the plastic bag. Small scissors should be used to cut the small roots.
In fact, the foodweb structure in any kind of material, from lake sediment, to rumen material from cattle can be assessed, but most research has been performed on soil-related material.
Interpretation of Soil Foodweb Structure
Ratio of total fungal to total bacterial biomass
By examining the structure of the soil foodweb in a range of soils, all grassland and most agricultural soils have ratios of total fungal to total bacterial biomass less than one (F/B < 1). Another way to interpret this is that the bacterial biomass is greater than the fungal biomass in these soils. In the most productive agricultural systems, however, the ratio of total fungal to total bacterial biomass equals one (F/B = 1) or the biomass of fungi and bacteria is even. When agricultural soils become fungal-dominated, productivity will be reduced, and in most cases, liming and mixing of the soil (plowing) is needed to return the system to a bacterial-dominated soil.
All conifer forest soils are fungal dominated, and the ratio in all forest soils in which seedling regeneration occurs is above 10. In general, productive forest soils have ratios greater than 100. This means that fungal biomass strongly outweighs the bacterial biomass in forest soils. In the case where forest soils lose this fungal-dominance, it is not possible to re-establish seedlings. When forest soil becomes bacterial-dominated, conifer seedlings are incapable of being re-established.
In the few studies of riparian forests that have been performed, some deciduous riparian forest soils are bacterial-dominated. In the case of riparian aspen and beech soils, the soils are bacterial dominated. But poplar, oak and maple soils are is fungal-dominated, although not as strongly fungal-dominated as in conifer systems. No studies on establishment of seedlings in these systems have been performed.
The ratio of total fungal to total bacterial biomass has been related to ecosystem productivity, but numbers or length of active and total bacteria and fungi are also indicative of the health of soil. For different soils, vegetation and climate, the density of bacteria or fungi indicate the past degradation of the soil. As explained above, and again in the following sections, bacterial numbers should be greater than one million for all agricultural soils, preferably nearer 100 million for the most productive soils. For the most productive forest soils, for example, fungal length should be above 5000 meters of hyphae per gram soil.
Biomass of total fungi
Fungal biomass is extremely important in all soils as a means of retaining nutrients that plants need in the upper layers of the soil, i.e., in the root-zone. Without these organisms to take-up nutrients, and either retain those nutrients in their biomass, or to sequester those nutrients in soil organic matter, nutrients would wash through the soil and into ground or surface water. Plants would suffer from lack of nutrients cycling into forms that the roots can take-up, if these nutrients aren't first immobilized in the soil through the action of fungi or bacteria. For forest soils, fungi sequester most of the nutrients, although significant portions are immobilized by bacteria as well.
In soil in which only fungi are present, the soil will become more acidic, from secondary metabolites produced by fungi. Aggregates are larger in fungal-dominated soils than in bacterial-dominated soils, and the major form of N is ammonium, since fungi do not nitrify N. These conditions are more beneficial for certain shrubs, and most trees.
Total fungal biomass varies depending on soil type, vegetation, organic matter levels, recent pesticide use, soil disturbance and a variety of other factors, many of which have not been researched completely. However, for normal grassland soils, total fungal biomass levels are usually around 50 to 500 meters per gram of soil. For agricultural soils, fungal biomass is around 1 to 50 meters per gram soil, while for forest soils, fungal biomass is between 1000 meters to 60 km per gram of soil. More work is necessary to establish what the optimal fungal biomass value should be for each type of crop, soil, organic matter, climate, etc. Very little information is available for tropical systems, but that small amount of data indicates that temperate systems perform very differently from tropical soils.
The average diameter of hyphae in most soils is about 2.5 micrometers, indicating typical mixtures of zygomycetes, ascomycete and basidiomycetes species. On occasion the average diameter may be greater than 2.5 micrometers, indicating a greater than normal component of basidiomycete hyphae, while on other occasions, the average diameter of hyphae may be less than 2.5 micrometers, indicating a change in species composition of soil fungi to a greater proportion of lower fungi. Actinomycetes are not usually differentiated from fungi, since actinomycetes are hyphal in morphology and are rarely of significant biomass. In some agricultural soils, these narrow diameter "hyphae" are of considerable importance, as demonstrated by Dr. A. Van Bruggan.
Biomass of active fungi
Activity in all soil organisms follow a typical seasonal fluctuation. This cycle is related to optimal temperature and moisture, such that a peak in activity usually occurs in the spring as temperature and moisture become optimal after cold winter temperatures. In systems where snow accumulates on the Coil surface, such that the soil does not actually freeze, fungal activity may continue at high levels throughout the winter in litter. Decomposition may continue at the highest rates through the winter under the snow in the litter. In systems where moisture becomes limiting in the summer, activity may reach levels even lower than in the winter. When temperatures remain warm in the fall and rain begins again after a summer drought, such as in Mediterranean climates, a second peak of activity may be observed in the fall. If these peaks are not observed, this suggests inadequate organic matter in the soil.
Numbers of total bacteria
Just as fungi are the most important players in retaining nutrients in forest soil, bacteria are the important players in agricultural and grassland soils. Bacteria retain nutrients first in their biomass, and second, in their metabolic by-products. In soil in which only bacteria are inoculated, the soil will become more alkaline, will have small aggregates, and generally will have nitrate/nitrite as the dominant form of N. These conditions are beneficial for grasses and row crop plants.
Numbers of total bacteria generally remain the same regardless of soil type or vegetation. Total bacterial numbers range between 1 million and 100 million per gram soil in agricultural soils, and between 10 million and 1,000 million in forest soils. Bacterial numbers can be above 100 million in decomposing logs, in anaerobic soils, in soil amended with sewage sludge or in soil with high amounts of comported material. In some instances following pesticide treatment, bacterial numbers can fall below 1 million, and this has been correlated with signs of severe nitrogen deficiency in plants. Bacterial numbers can drop to extremely low levels, below 100,000 per gram of soil, in degraded soils where nutrient retention is a problem.
Biomass of active bacteria
As with active fungal biomass, bacterial activity usually peaks in the spring and decreases during the summer with drought. If the temperature remains warm in the fall and fall rains begin, a second peak of activity usually occurs. The ratio of active fungal to active bacterial biomass, even in forests, shows that bacterial biomass is usually more active than fungal biomass. If these peaks with temperature and moisture are not seen, then lack of appropriate food in the soil to support bacterial and fungal biomass is suspected. If bacterial and fungal activity does not respond to seasonal fluctuations, then subsequent impacts on the predators in the soil will be observed.
Protozoan numbers
Protozoa feed on bacteria, and as they feed on their prey, N is released. It's unclear just how much N is released per individual feeding event, since it depends on whether the bacterium was actively growing, thus containing more N. or whether the bacterium was in a resting starving phase, and containing much less N. Several studies have shown that a major portion t40-80%) of the nitrogen that cycles through in certain agricultural soils is cycled by protozoa. Without these organisms in soil, plants suffer a significant reduction in available N. However the optimal relationship between the number of bacteria and the number of protozoa has not been quantified.
There appears to be a great range in protozoan numbers from soil to soil, and even from field to field. Some of the observations that have been made, when dealing with agricultural soil tie., bacterial dominated) is that when protozoan numbers are high, bacterial-feeding nematode numbers will be low, and vice versa. Thus there appears to be significant competition between bacterial-feeding predators for the bacterial prey. Whether this is indicative of the type of bacteria present in the soil, and whether this has any relationship to productivity in agricultural situations is not known. Testate amoebae are only found in significant and constant numbers in forest soils, and are never found in temperate agricultural soils. Why this is the case is not known, but continues to be observed.
Nematode numbers, community structure
Nematode handout
There are four major types of nematodes, which includes bacterial-feeding, fungal-feeding, root-feeding and predatory nematodes. All nematodes are predators, and thus reflect to some extent the availability of their prey groups. However, other organisms prey upon these nematodes as well, and nematode numbers can also reflect the balance between the availability of nematode prey, as well as feeding by nematode predators.
Both bacterial-feeding and fungal-feeding nematodes mineralize N from their prey groups. Bacterial-feeding nematodes are more important in bacterial-dominated soils (agriculture and grassland systems), while fungal-feeding nematodes are more important in fungal dominated soils (conifer and most deciduous forests). Between 70 and 80% of the nitrogen in rapidly-growing trees has been shown to come from interactions between nematode predators and their prey. Between 30 and 50% of the N in crop plants appears to come form the interactions of bacterial-feeding nematodes and bacteria. Thus, the presence and numbers of bacterial- and fungal-feeding nematodes is extremely important for productive soils.
Root-feeding nematodes are detrimental to plant growth. As few as one endo-parasitic nematodes per plant may be enough to result in decreased productivity or death, while plants may tolerate several hundred ecto-parasitic nematodes per root system without reduction in production. Compensatory plant production has been observed with a little root-feeding, in that plant production is greater with a few herbivores munching on the plant than without feeding taking place.
Root-feeding nematode numbers can be reduced by competition for root space. VA mycorrhizal fungi may prevent root-feeding nematodes from reaching the roots through a variety of mechanisms. Nematode trapping fungi trap and kill many root-feeding nematodes. Other fungi and bacteria may be active inhibitors of nematode presence in the rhizosphere. Effective biocontrol of these plant-feeders is being worked on and may be possible in the near future.
T. Bongers, in the Netherlands, suggested the use of a Maturity Index for nematodes in soil. Certain species of nematodes are more commonly found following disturbance, while other species are more typical inhabitants of less-disturbed soils. The numbers of the four different trophic groups, or of different genera or species of matodes can be interpreted in several ways. First, significant differences in the number of individuals in a trophic group, or in nematode species in any treatment, compared to the control or reference treatment indicates an impact on that particular group or species. Several studies have recently shown that changes in numbers of even a single species of nematode in soil can significantly alter nutrient cycling within a soil. However, until more work is done to determine whether the same (or similar) nematode species control that same process in a similar way in other soils, extrapolation of results from a study performed in a different soil, with different plant species, different bacterial and fungal species, and different numbers of competing predators remains fraught with difficulty. However, it clearly suggests that alteration in nematode species composition could have negative impacts on plant growth, plant species composition, and thus ecosystem productivity.
A second way of interpreting nematode data is to assess changes in process rates. Since a bacterial-feeding nematode consumes 106 bacteria per day, reductions in this trophic group should result in an increase in bacterial biomass, with concomitant increase in net nutrient immobilization, and a decrease in nutrient mineralization with a concomitant effect on availability to plants. A fungal-feeding nematode consumes the cytoplasm in 10-50 meters of hyphal length per day, with similar effects on nutrient cycling as decreases in bacterial-feeding nematodes.
When root-feeding nematodes numbers are decreased, the ecosystem impact is positive, since root-feeding nematodes reduce plant growth/yield. However, root-feeding nematodes are highly opportunistic organisms, and are among the first organisms to invade after disturbance. Thus, one result of any disturbance which seriously affects ecosystem stability is reduction in the number of organisms which displace root-feeding nematodes. These competitors of root-feeding nematodes are mycorrhizal fungi, nematode-feeding nematodes, nematode-feeding microarthropods which frequent the rhizosphere, and fungal-feeding nematodes which apparently interfere with the ability of root-feeding nematodes to find roots. A second result of disturbance is a reduction in the ability of plants to resist nematode feeding. Thus, following a disturbance which disrupts ecosystem stability, root-feeding nematode numbers increase more rapidly than other groups, to the detriment of ecosystem productivity. Thus, as a general indicator of ecosystem health, increases in root feeding nematode numbers suggest serious negative impacts on ecosystem stability.
A decrease in nematode-feeding nematode numbers initiates a trophic cascade effect. For example, nematode-feeding nematodes can control the populations of bacterial-, fungal- or root-feeding nematodes and reduction in nematode-feeders results in an immediate increase in their prey group - i.e., bacterial- fungal- or root feeding nematodes. This in turn results in a decrease in the prey of these three nematode groups; a reduction in bacteria, fungi or roots, each with it's detrimental effect on nutrient cycling Indoor plant growth, as outlined above.
VAM spore numbers
Vesicular-arbuscular mycorrhizal (VAM) fungi are critically important for all crop plants, except species of the brassica family (e.g., mustards, kale). A number of researchers have shown that the lack of VAM inoculum, or the lack of the appropriate inoculum can result in poor plant growth, in poor competition with other plants or inability to reproduce or survive under certain extreme conditions. However, most crop fields have adequate VAM spores present, especially if crop residue is placed back into the field. Only in a few situations where soil degradation has been severe, such as with intensive pesticide use, fumigation, or intense fertilizer amendment, will VAM inoculum become so low that plant growth will be in jeopardy.
In restoration studies, the lack of appropriate inoculum is more likely to be a problem than in other situations where sources of appropriate VAM spores are near-by. Thus, the presence of at least 1 to 5 spores per gram of soil is adequate for most crop fields. When the number of spores falls below one per gram, then addition of compost containing high numbers of VAM spores (for example from an alfalfa field, or other legume), or inoculation of VAM spores from a commercial source generally results in positive effects.
Percent VAM colonization
At least 12% of the root system of grasses, (i.e., most crop plants), should be colonized by VAM in order to obtain the minimum required benefits from this symbiotic relationship. Colonization upwards of 40% is usually seen in healthy soils. VAM colonization can limit root-feeding nematode attack of root systems, if the nematode burden is not too high. A great deal knowledge of the relationship between plant species, VAM species and soil type, including fertility, is needed in order to fully predict the optimal relationship between crop plant, VAM species and soil. For more information about the Soil Microbial Biomass Service and how to submit a soil sample, write Dr. Elaine Ingham, Department of Botany and Plant Pathology, Cordley Hall 2082, Oregon State University, Corvallis, OR 97331-2902.
References
Allen, M.F. and E.B Allen. 1992. Mycorrhizae and plant community development: Mechanisms and patterns. pp. 455-480. IN Carroll, G.and D.T. Wicklow (eds). The Fungal Community: Its Organization and Role in the Ecosystem. Marcel DeRker, New York.
Allen, M.F. 1992. Mycorrhizal Functioning.New York Chapman and Hall, Inc.
Bongers, T. 1988. De nematoden van nederland. Pirola Schoorl. Natuurhist. Biblioth. KNNV nr. 46. Wageningen Agricultural University, The Netherlands.
Bongers, T. 1990. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 83: 14-19.
Coleman, D.C., E.P.Odum, and D.A. Crossley, Jr. 1992. Soil biology, soil ecology and global change. Biol. Fert. Soils 14:104-111.
Coleman, D.C., 1985. Through a ped darkly: An ecological assessment of root-soil-microbial-fauna! interactions. In: A. H. Fitter, D. Atkinson, D.J. Read, and M.B. Usher (Editors), Ecological Interactions in Soil. Blackwell Scientific Publications, Cambridge, U.K. pp. 1-21.
Colinas et al, 1993
De Goede, R.G.M., S.S. Georgieva, B.C. Verschoor and J. Kamerman. 1993. Changes in nematode community structure in a primary succession of blown-out areas in a drift sand landscape. Fundamental and Applied Nematology 16: 501-513.
Dindal, D. 1990. Soil Biology Guide. John Wiley and Sons. 1349 pp.
Ettema, C.H. and T. Bongers. 1993. Characterization of nematode colonization and succession in disturbed soil using the maturity index. Biology and Fertility of Soils 16: 79-85.
Foissner, W. 1986. Soil protozoa: fundamental problems, ecological significance, adaptations, indicators of environmental quality, guide to the literature. Prog. Protist. 2:69-212.
Hendrix, P.F., R.W. Parmelee, D.A. Crossley, Jr., D.C. Coleman, E.P. Odum, and P.M. Groffman. 1986. Detritus foodwebs in conventional and no-tillage agroecosystems. Bioscience 36:374-380.
Ingham, E.R. and D.C. Coleman. 1984. Effects of streptomycin, cycloheximide, fungizone, captan, carbofuran, cygon, and PCNB on soil microbe populations and nutrient cycling. Microbial Ecol 10:345-358.
Ingham E.R. and Horton K.A. 1987.Bacterial, fungal and protozoan responses to chloroform fumigation in stored prairie soil. Soil Biology & Biochemistry 19:545-550.
Ingham, E.R., J.A. Trofymow, R.N. Ames, H.W. Hunt, C.R. Morley, J.C. Moore, and D.C. Coleman. 1986a. Trophic interactions and nitrogen cycling in a semiarid grassland soil. Part I. Seasonal dynamics of the soil foodweb. J. Appl. Ecol. 23:608-615.
Ingham, E.R., J.A. Trofymow, R.N. Ames, H.W. Hunt, C.R. Morley, J.C. Moore, and D.C. Coleman. 1986b. Trophic interactions and nitrogen cycling in a semiarid grassland soil. Part II. System responses to removal of different groups of soil microbes or fauna. J. Appl. Ecol. 23:615-630.
Ingham, E.R., D.C. Coleman, and J.C. Moore. 1989. An analysis of food-web structure and function in a shortgrass prairie, a mountain meadow, and a lodgepole pine forest. Biol. Fert. Soils 8:29-37.
Ingham, E.R. W.G. Thies, D.L. Luoma, A.R. Moldenke and M.A. Castellano. 1991. Bioresponse of non-target organisms resulting from the use of chloropicrin to control laminated root rot in a Northwest conifer forest: Part 2. Evaluation of bioresponses. pp. 85-90. IN USEPA Conference Proceedings. Pesticides in Natural Systems: Can Their Effects Be Monitored? USEPA Region 10, Seattle, WA.
Ingham, E.R. and W. Thies. 1995. Soil foodweb responses following disturbance: Effects of clearcutting and application of chloropicrin to Douglas-fir stumps. Applied Soil Ecology. in press.
Ingham, R.E., J.A. Trofymow, E.R. Ingham, and D.C. Coleman. 1985. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecol. Monogr. 55:119-140.
Ingham, R.E. 1992. Interactions between invertebrates and fungi: Effects on nutrient availability. pp. 669-690. In G.C. Carroll and D.T. Wicklow (Eds). The Fungal Community: Its Organization and Role in the Ecosystem, Second Edition, Marcel Dekker, New York.
Kilham, K. 1994. Soil Ecology. Cambridge University Press, Combridge, UK.
Kuikman, P.J., Van Elsas, J.D., A.G. Jassen, S.L.G.E. Burgers and J.A. Van Veen. 1990. Population dynamics and activity of bacteria and protozoa in relation to their spatial distribution in soil. Soil Biol. Biochem. 22:1063-1073.
Klopatek, et al. 1993
Lal, B. and J.P. Stewart. 1992. Soil Restoration. Spri~nger-Verlag, New York, N.Y. 349 pp.
Moore, J.C., H.W. Hunt and E.T. Elliott. 1991. Ecosystem properties,soil organisms and herbivores. p. 105-140. IN P. Barbosa, V.A. Krischik, and C.G. Jones. 1991. Microbial Mediation of PlantHerbivore Interactions. John Wiley & Sons, Inc. New York, NY
Nannipieri, P., S. Grego, and B. Ceccanti. 1990. Ecological significance of the biological activity in soil. Soil Biochemistry 6:293-355.
Nicholas, W.L. 1975. The biology of free-living nematodes. Clardon Press, Oxford.
Paul, E.A and Clark F.E. 1990. Soil Microbiology and Biochemistry. Academic Press, Inc. San Diego, CA. 273 pp.
Perry, D.A., M.P. Amaranthus, J.G. Borchers, S.L. Borchers and R.E. Brainerd. 1989. Bootstrapping in ecosystems, BioScience 39:230-237.
Yeates, G.W., S.S. Bamforth, D.J. Ross, K.R. Tate and G.P. Sparling. 1991. Recolonization of methyl bromide sterilized soils under four different field conditions. Biology and Fertility of Soils 11: 181189.
Yeates, G.W., T. Bongers, R.G.M. De Goede, D.W. Freckman and S.S. Georgieva. 1993. Feeding habits in nematode families and genera - an outline for soil ecologists. Jr. of Nematology 25: 315-331.
Woods, L.E., C.V. Cole, E.T. Elliott, R.V. Anderson and D.C. Colemar 1982. Nitrogen transformations in soil as affected by bacterialmicrofaunal interactions. Soil Biol. Biochem. 14:93-98.
 Australian Dollars
Australian Dollars
 US Dollars
US Dollars
 European Euros
European Euros
 New Zealand Dollars
New Zealand Dollars